By Medeea GreereMay 8, 2024Updated:May 8, 2024No Comments5 Mins Read
TwitterFacebookTelegramWhatsApp
SHARE
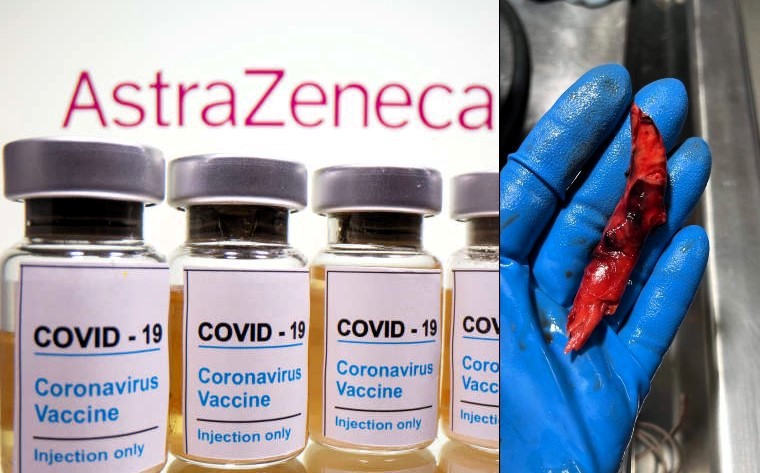
Breaking News: AstraZeneca to Withdraw COVID Vaccine Globally – The Fall of a Pandemic Titan
Medeea Greere, an independent publisher, is now on Telegram at https://t.me/AMGNEWS2022 and exists only on reader support as we publish Truth, Freedom and Love for public awareness. Thank You for your support!
AstraZeneca has pulled the plug on its once-celebrated COVID-19 vaccine, Vaxzevria. The company cites a steep decline in market demand as its reason, yet lurking beneath the surface are significant legal battles tied to severe blood clotting incidents. Is this truly a business decision, or is AstraZeneca retreating amid rising legal storms?
The Unexpected End of Vaxzevria
It was an announcement that shook the pharmaceutical industry to its core. AstraZeneca, a giant in the world of vaccine development, declared the cessation of its COVID-19 vaccine, Vaxzevria, on a global scale. This decision, ostensibly made due to plummeting demand, intersects suspiciously with a barrage of legal challenges and mounting evidence linking the vaccine to rare but grave blood clot complications.
The Shadows Behind the Decision
Market demand is a plausible façade, yet the reality might be more intricately tied to the legal nightmares unfolding in courts worldwide. As the lawsuits pile up, each claiming that the vaccine led to serious health issues, the question arises: Has AstraZeneca opted for retreat to mitigate further reputational damage? This is a question that not only invites scrutiny but demands a deep dive into the complex interplay of medicine, law, and corporate strategy.
Shocking Secret EXPOSED: Ex-Big Pharma Employee Discovers Breakthrough Formula: EAT THIS ONCE A DAY TO LOWER BLOOD SUGAR BELOW 100 ALL DAY
Vaxzevria: A Pillar of Early Pandemic Response
At the pandemic’s zenith, Vaxzevria was hailed as a beacon of hope. Its development was a race against time, and upon its release, it was quickly distributed worldwide, offering protection against the ravaging effects of COVID-19. Millions received the jab, and infection rates in early vaccine adopter nations showcased a significant drop, crediting Vaxzevria with a critical role in their pandemic strategy. The vaccine’s impact was undeniable, lauded for its role in saving countless lives.
The Tidal Wave of Legal Challenges
However, the rising tide of legal disputes paints a starkly different picture. Allegations of severe adverse effects, particularly blood clots, began to tarnish its image. These weren’t just minor grievances; they were serious, life-threatening conditions that prompted global concern. Health agencies and regulatory bodies faced pressure to reassess the vaccine’s safety profile, leading to a spectrum of responses, from heightened warnings to outright bans in some countries.
Medicinal Garden Kit: Your Secret Arsenal Against Big Pharma and Ailing Health

The Market’s Cold Shoulder
As confidence waned, so did demand. The correlation between emerging legal battles and the declining market interest suggests a causative link that AstraZeneca vehemently denies. Yet, the timing of these events—legal escalations followed closely by the withdrawal announcement—raises eyebrows and questions about the real motives behind this decision. Could this be a strategic retreat, cloaked in the guise of responding to market dynamics?
The Reputational Repercussions
The fallout from this withdrawal is multi-faceted. Not only does it impact AstraZeneca’s standing in the pharmaceutical community, but it also shakes the public’s confidence in vaccination campaigns. The echoes of this decision will undoubtedly influence how vaccines are perceived and received in the future, potentially fueling vaccine hesitancy.
The Future of Vaccination and Legal Accountability
This withdrawal sets a precedent for how pharmaceutical companies may respond to similar crises in the future. It underscores the delicate balance between public health interests and the immense pressures facing vaccine manufacturers, both legally and commercially. As AstraZeneca steps back from Vaxzevria, the global health community must navigate the repercussions and ensure that vaccine development continues to advance, albeit under the ever-watchful eye of public and legal scrutiny.
Conclusion
The discontinuation of Vaxzevria is a significant chapter in the ongoing saga of COVID-19. It serves as a potent reminder of the complex challenges that emerge at the intersection of health science and legal liability. As the world grapples with these dynamics, the story of Vaxzevria will likely be remembered as a cautionary tale of what can happen when public health initiatives are caught in the crossfire of legal and commercial battles. AstraZeneca’s move might be seen as a surrender, a strategic calculation, or a necessary step back, but regardless of perspective, it marks a pivotal moment in the ongoing fight against global health crises.
Avi Yemini: When I met the AstraZeneca boss in Davos, he claimed Covid vaccine mandates were needed to PROTECT as many people as possible. Today, his drug was pulled off the market after it was revealed it HURT the same people forced to have it. Let that sink in.





